Eurobio Oncology
EndoPredict®: Local Testing
EndoPredict®
Fast, Reliable, and Decentralized
EndoPredict is a second-generation gene expression test for ER+ HER2- breast cancer, developed specifically for use in local pathology laboratories. It provides both prognostic and predictive insights1-3, supporting confident chemotherapy decisions with high analytical4 and clinical accuracy.5,6
- Available as a CE-IVD kit, EndoPredict is validated for routine use in molecular pathology labs and is already established in over 30 countries and more than 90 local laboratories worldwide.
- Installation and qualification at a new site takes just two days, guided by Eurobio’s experienced customer support team. This process ensures consistent, reproducible results – independent of where the test is performed.
By enabling local testing, EndoPredict allows clinicians to access results quickly, without the delays or logistical challenges of sending samples abroad.
Highly Reproducible in Local Laboratories
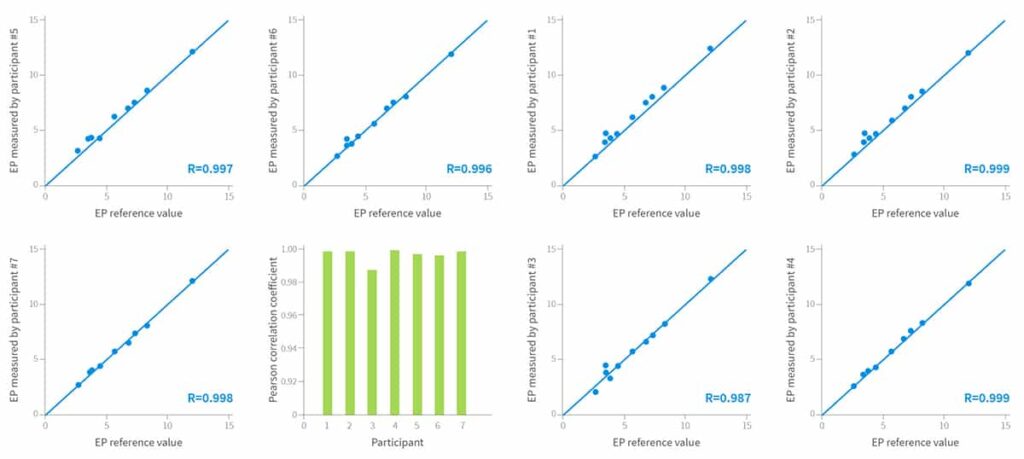
EndoPredict consistently delivers the same quality of results, regardless of the pathological institute conducting the assay, as has been demonstrated in round-robin testing.7-9
The variance and accuracy of the EndoPredict assays were determined using predefined reference values.
Evaluation of the assay in 7 different molecular pathology laboratories in Germany, Austria, and Switzerland8 showed:
- Exceptional correlation with reference values – mean value of Pearson correlation coefficients, r= 0.996
- All samples were assigned to the correct EndoPredict risk group, resulting in:
- Sensitivity and specificity of 100%
- Concordance of 100%
- Kappa of 1.0
Results of the decentral measurement of 70 tumor samples from ten different tumors7
Flexible Testing – Surgical Specimens and Biopsies
EndoPredict is validated for use on both surgical tumor specimens and core needle biopsies, ensuring maximum flexibility in clinical practice. This allows physicians to obtain reliable prognostic and predictive information at different stages of the patient journey – whether before surgery, to discuss individual therapy options with patients as soon as possible, or after surgery, to guide adjuvant treatment planning.
A comparison of test results between core biopsies and corresponding surgical breast cancer sections, analyzing the influence of biopsy-related tissue injury on the test result, revealed a high correlation between the paired samples. The molecular score was highly correlated between biopsies and tumour sections (r=0.92), and the concordance for the EndoPredict-based risk classification was excellent (overall agreement 95%, k=0.89).10
By enabling testing on both sample types, EndoPredict delivers consistent, high-quality results with the same proven analytical and clinical accuracy.
EndoPredict® Testing – Fast, Local, Reliable
The EndoPredict prognostic and predictive breast cancer test is performed in certified local laboratories, delivering reliable results within just a few days.
Physicians can conveniently order EndoPredict through a wide network of participating labs.
Use the link below to find a local lab and place your order. Have questions? Contact us anytime – we’re here to help.

Get the EndoPredict® Test in Your Lab
EndoPredict is available as a CE-marked IVD kit that can be run in local molecular pathology laboratories after a comprehensive onsite training, provided by Eurobio Scientific.
Interested in offering EndoPredict in your lab? Use the contact form below – we’ll evaluate the requirements together.
References
- Filipits M. et al.: A New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk Factors. Clin. Cancer Res. 2011
- Sestak I. et al.: Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Res Treat. 2019
- Filipits M. et al.: Prediction of Distant Recurrence using EndoPredict among Women with ER+, HER2- Node- Positive and Node-Negative Breast Cancer Treated with Endocrine Therapy Only. Clin Cancer Res. 2019
- EndoPredict® QS for QuantStudio™ 5 Dx Instruction Manual Version EN/03 2025
- Buus R. et al.: Comparison of EndoPredict and EPclin With Oncotype DX Recurrence Score for Prediction of Risk of Distant Recurrence After Endocrine Therapy. J Natl Cancer Inst. 2016
- Sestak I. et al.: Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer. A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018
- Kronenwett R. et al.: Decentral gene expression analysis: analytical validation of the EndoPredict genomic multianalyte breast cancer prognosis test. BMC Cancer. 2012
- Denkert C. et al.: Decentral gene expression analysis for ER+/Her2- breast cancer: results of a proficiency testing program for the EndoPredict assay. Virchows Arch. 2012
- Lehmann-Che J. et al.: First French pilot quality assessment of the EndoPredict test for early luminal breast carcinoma. Anticancer Res. 2018
- Muller B. M. et al.: The EndoPredict Gene-Expression Assay in Clinical Practice – Performance and Impact on Clinical Decisions. PloS ONE. 2013