Une précision fiable – dans votre laboratoire ou via notre réseau
Realizado localmente, accesible globalmente
- EndoPredict es una prueba de expresión génica de segunda generación diseñada para detectar la recurrencia del cáncer de mama precoz ER-positivo HER2-negativo (ER+/HER2-). Ofrece una mayor precisión al combinar datos moleculares con factores clínico-patológicos.1,4 Respaldado por evidencia de Nivel 1A en ESMO1 y recomendada por las principales directrices,13-17 EndoPredict favorece la toma de decisiones informadas que reducen el sobretratamiento y su impacto a largo plazo en la vida de los pacientes.
Disponible como kit IVD con marcado CE para laboratorios de patología autorizados en numerosos países, puede utilizarse localmente tras recibir formación y obtener la cualificación, o bien es accesible a través de nuestra red global de más de 90 laboratorios asociados, lo que garantiza resultados rápidos y fiables para médicos y pacientes.
Los médicos confían en EndoPredict®
EndoPredict es una prueba genómica avanzada que los patólogos pueden recomendar para ayudar a planificar un tratamiento personalizado para el cáncer de mama ER+/HER2- en fase inicial. Los médicos confían en EndoPredict por su fiabilidad y su precisión pronóstica y predictiva, lo que la convierte en una herramienta valiosa en la práctica diaria.
Con sólo 2,5 horas de práctica y un tiempo total de respuesta de aproximadamente 8 horas, la prueba se adapta perfectamente a los flujos de trabajo de los laboratorios moleculares, al tiempo que garantiza una rápida entrega de los resultados a los médicos tratantes.
Desbloquear la precisión: qué es EndoPredict® Revela en sus resultados
- Riesgo individual a 10 años de recurrencia a distancia1
- Probabilidad individual de beneficio de la quimioterapia en 10 años2
- Riesgo individual de recidiva tardía a distancia entre los años 5 y 153
Descubra la información detallada que proporciona el informe de resultados de EndoPredict. Explore la estructura del informe, interprete las puntuaciones de riesgo y comprenda cómo esta información respalda las decisiones de tratamiento personalizadas.

Pruebas de segunda generación: mayor fiabilidad y claridad
EndoPredict es una prueba de expresión génica de segunda generación desarrollada para ofrecer una mayor precisión pronóstica y predictiva que permita tomar decisiones terapéuticas informadas.4,5
Por qué es importante la segunda generación: EndoPredict va más allá del simple perfil molecular. Combina:
- Puntuación molecular de 12 genes - Capta el riesgo de recidiva a distancia temprana y tardía1,18
- Factores clinicopatológicos - Integra el tamaño del tumor y el estado ganglionar directamente en el resultado1
- Kit CE-IVD para pruebas locales - Permite una respuesta rápida y una calidad controlada por el laboratorio en los flujos de trabajo de patología molecular.

Los patólogos pueden tomar decisiones fiables y conformes con las directrices gracias a una prueba reconocida por médicos de todo el mundo.
Para comprender cómo EndoPredict integra datos moleculares y clínicos con el fin de proporcionar un pronóstico preciso, le invitamos a explorar nuestra aplicación animada interactiva.
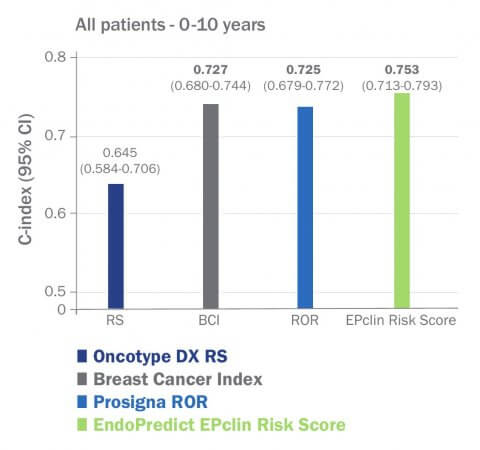
Capacidad pronóstica para detectar recidivas a distancia5
El índice C es una estadística estándar utilizada para medir la precisión pronóstica. Permite comparar diferentes pruebas independientemente de sus valores umbral. Cuanto mayor sea el índice C, mayor será el rendimiento pronóstico de una prueba.Superioridad probada en la estratificación de riesgos
- Más información sobre la precisión superior de EndoPredict
Pruebas fiables
Recomendado en las principales directrices internacionales, entre ellas las de la ASCO, la NCCN, la AJCC, St. Gallen y el NICE, así como en la recomendación de nivel de evidencia 1A de la ESMO.12-17 Además, ocupa un lugar destacado en las directrices nacionales de todo el mundo.
Recomendación ESMO LoE1A para enfermedades sin afectación ganglionar y con afectación ganglionar12
EndoPredict está reconocido en las directrices actuales sobre cáncer de mama como una prueba destinada a pacientes en pre y posmenopausia. Las directrices excluyen los resultados de riesgo intermedio, lo que confiere a EndoPredict una clara ventaja, con solo categorías de riesgo bajo y alto para tomar decisiones fiables sobre la quimioterapia.
Máximo nivel de evidencia (LoE1A)
Inclusión de pacientes premenopáusicas
Todos los pacientes incluidos debido a resultados claros

Cómo solicitar un EndoPredict® Pruebas para su paciente
La prueba EndoPredict, que permite predecir el pronóstico del cáncer de mama y la respuesta a la quimioterapia, está disponible para los médicos en numerosos laboratorios locales.
- Encuentre su laboratorio local
Le invitamos a seleccionar y ponerse en contacto con su laboratorio de análisis médicos local de nuestra lista de laboratorios certificados que realizan la prueba EndoPredict. Trabajar con un laboratorio cercano a usted garantiza una entrega más rápida de los resultados.
- Envíe su muestra
Las muestras tumorales procedentes de una biopsia diagnóstica o de una muestra quirúrgica (antes del tratamiento) pueden enviarse directamente al laboratorio elegido. El laboratorio le proporcionará instrucciones detalladas para la preparación y el envío de las muestras.
- Resultados en días
Una vez que el laboratorio recibe la muestra, los resultados suelen estar disponibles en pocos días, lo que le permite tomar rápidamente decisiones terapéuticas informadas para sus pacientes.
Cómo solicitar un EndoPredict® Kit
Los patólogos ya formados y cualificados para utilizar EndoPredict en sus laboratorios pueden solicitar los kits directamente. Tenga en cuenta que los kits solo están disponibles para laboratorios con una implementación consolidada y personal cualificado.
Obtenga el EndoPredict® Pruebas en su laboratorio
EndoPredict está disponible como un kit de DIV con marcado CE que puede utilizarse en laboratorios locales de patología molecular tras una formación exhaustiva in situ impartida por Eurobio Scientific.
¿Desea ofrecer EndoPredict en su laboratorio? Le invitamos a utilizar el formulario de contacto que aparece a continuación; examinaremos juntos los requisitos necesarios.
Referencias
- Filipits M. et al.: A New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk Factors. Clin. Cancer Res. 2011
- Sestak I. et al.: Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Res Treat. 2019
- Filipits M. et al.: Prediction of Distant Recurrence using EndoPredict among Women with ER+, HER2- Node- Positive and Node-Negative Breast Cancer Treated with Endocrine Therapy Only. Clin Cancer Res. 2019
- Buus R. et al.: Comparison of EndoPredict and EPclin With Oncotype DX Recurrence Score for Prediction of Risk of Distant Recurrence After Endocrine Therapy. J Natl Cancer Inst. 2016
- Sestak I. et al.: Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer. A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018
- Constantinidou A. et al.: Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2022
- Sestak I. et al.: Prognostic Value of EndoPredict in Women with Hormone Receptor Positive, HER2-Negative Invasive Lobular Breast Cancer. Clin Cancer Res. 2020
- Klein, E. et al.: Long-term outcome data using EndoPredict as risk stratification and chemotherapy decision biomarker in hormone receptor positive, HER2-negative early breast cancer. Breast Cancer Res Treat. 2024
- Schmitt, W. D. et al.: Retrospective evaluation of outcomes in a real-world, prospective cohort using EndoPredict: Results from the Charité registry. SABCS 2022
- Vázquez-Juarez D. et al.: Seguimiento de cohorte prospectiva de mujeres premenopáusicas mexicanas con cáncer de mama que recibieron tratamiento adyuvante guiado con el ensayo EndoPredict. SABCS 2021
Jung et al: Treatment Outcomes according to the EndoPredict Score in ER-Positive, HER2-Negative Early Breast Cancer. Breast Care 2022
Loibl S. et al.: Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023
- Andre F. et al: Biomarcadores para el tratamiento adyuvante endocrino y quimioterápico del cáncer de mama en estadios tempranos: ASCO Guideline Update. J Clin Oncol. 2022
- Curigliano G. y otros: Comprender la complejidad del cáncer de mama para mejorar los resultados de las pacientes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 2023
- AJCC Cancer Staging Manual- Eight Edition 2017
- Tomado con permiso de NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer r V.4.2024 ©National Comprehensive Cancer Network, Inc. 2024. Todos los derechos reservados. Consultado el [17 de julio de 2024]. Para ver la versión más reciente y completa de la guía, visite NCCN.org.
- National Institute for Health and Care Excellence (2024). Tumor profiling tests to guide adjuvant chemotherapy decisions in early breast cancer (Diagnostics guidance [DG58]).
- Dubsky P. et al: The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013

