Clinicians
Confidence in Every Decision
Using EndoPredict delivers clarity – predictive, prognostic, and backed by Level 1A ESMO evidence.1-9
EndoPredict®
One Test – Three Clinical Answers
Accurate Risk Classification and Fast, Reliable Results
- Offers clear risk classification into low or high-risk categories
- Identifies the highest number of women with true low-risk in head-to-head comparisons with competitors5,6
- Delivers fast results through local testing for quicker decision-making
10-Year Recurrence Risk1
EndoPredict provides an accurate individual assessment for risk of breast cancer distant recurrence within 10 years, allowing you and your patients to make confident systemic therapy decisions together.
Chemotherapy Benefit2
EndoPredict predicts the individual chemotherapy benefit for women with ER positive/HER2 negative early-stage breast cancer, so confident treatment decisions can be made with your patients.
5-15-Year Recurrence Risk3
EndoPredict is the only test able to predict risk of distant recurrence up to 15 years, allowing you to predict the future and make safe choices with your patients today.
Second Generation Testing - More Accuracy for Your Patients1,5,6
EndoPredict is a second-generation gene expression test designed to deliver highly reliable guidance for treatment decisions.
Why Second-Generation Matters
Unlike first-generation tests, EndoPredict provides a more accurate and actionable risk assessment by integrating all of the following:
- 12-Gene Molecular Score – Genes that predict early and late metastasis
- Clinicopathological Factors – Incorporating tumor size and nodal status
- Local Testing with a CE-IVD Kit – Run in molecular pathology labs for faster results

12-Gene Molecular Score and EPclin Risk Score
Proven Superiority5
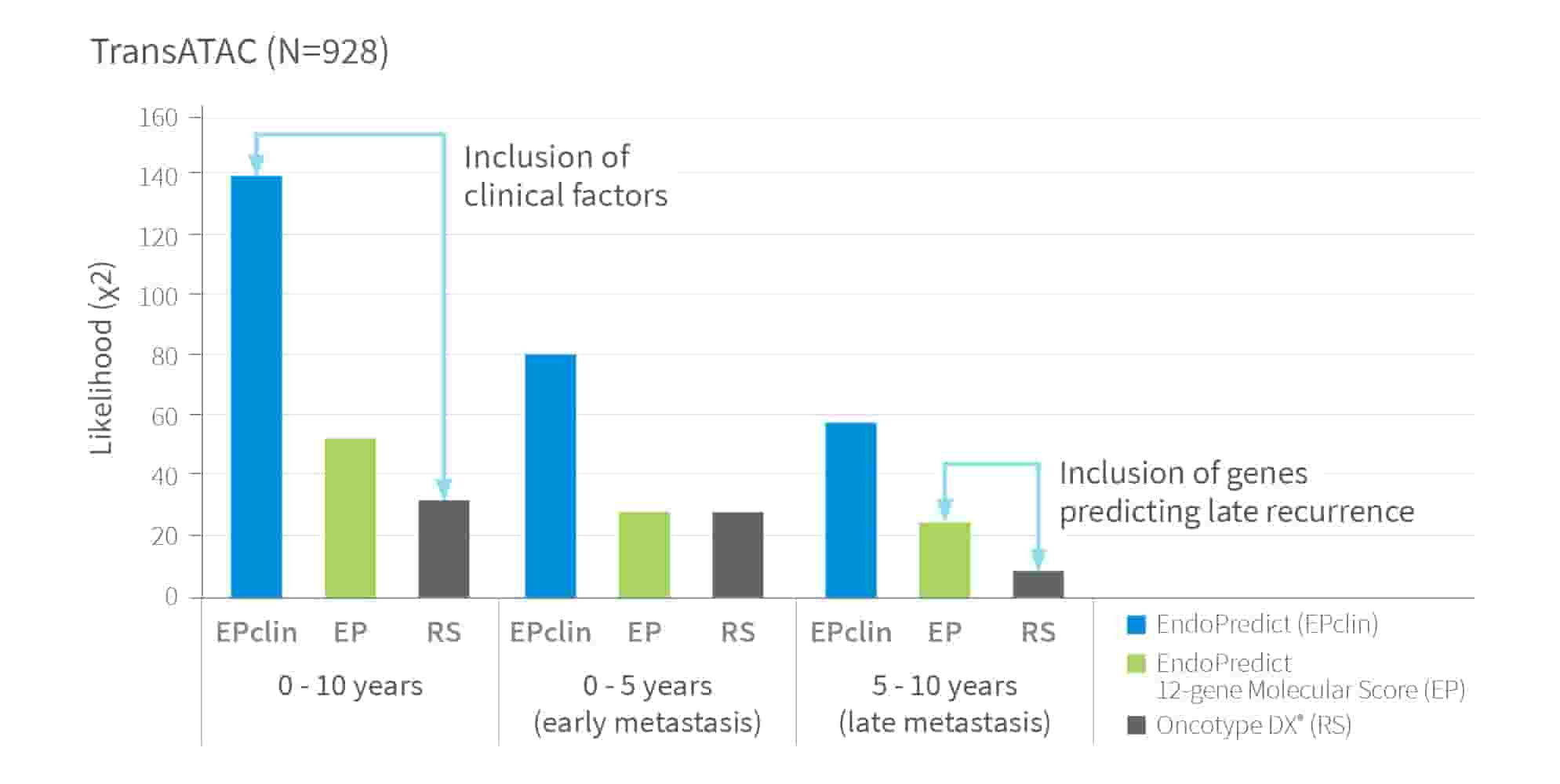
In the TransATAC study, EndoPredict outperformed the leading first-generation test in predicting recurrence risk independent from cut-off values. This ensures greater confidence in identifying patients who may benefit from chemotherapy.
Prognostic ability of EndoPredict, 12-Gene Molecular Score and RS to detect Distant Recurrence5
The X²-value is a standard measure of prognostic power. It allows comparison of different tests independent of cut-off values – with higher X²-values indicating stronger prognostic accuracy.
Advanced gene selection y well-balanced algorithm along with integrated clinical-pathological factors provide superior accuracy.
Reliable Risk Assessment and Chemotherapy Benefit Prediction Across all Patient Groups2-3,5-10
EndoPredict demonstrated reliability with consistent results across all studies and all patient populations, accurately identifying low-risk patients with a truly low-risk for distant metastasis.
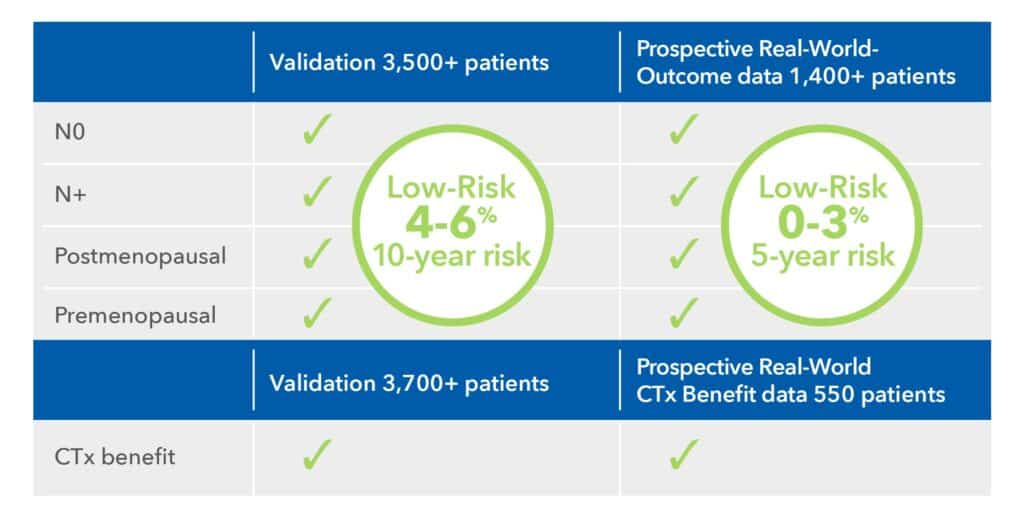
- Both validation studies and prospective real-world data confirm its ability to predict chemotherapy benefit, offering valuable insights to guide treatment decisions and improve patient outcomes.
Eligibility for EndoPredict® Testing1-8
EndoPredict provides essential insights to guide treatment decisions for women with invasive primary breast cancer who meet the following criteria:
- HER2 negative
- ER positive
- IDC (ductal) or ILC (lobular)
- Lymph node positive* or negative
- Tumor size pT1 to pT3
- Grade 1 to 3
- Pre- or postmenopausal
*It is recommended that EndoPredict is used only for patients with up to three positive lymph nodes
- EndoPredict is the only second-generation breast cancer recurrence test validated for both premenopausal and postmenopausal women, ensuring comprehensive treatment guidance across different patient groups. The test can be performed on FFPE tumor tissue from biopsy or surgical specimens prior to systemic therapy.
Lymph Node Negative and Positive
Does Nodal Status Indicate Breast Cancer Distant Recurrence Risk?
While nodal status is an important factor in determining prognosis, it doesn’t always reflect the full risk of distant recurrence. Not all patients with node-negative breastcancer are low-risk, and not all patients with node-positive disease are high-risk. For more accurate risk stratification, EndoPredict provides a clearer picture of distant recurrence risk, enhancing treatment decision-making.
EndoPredict is Validated for Node Negative and Node Positive Breast Cancer3
EndoPredict has been validated in large, pivotal clinical trials such as ABCSG6 and ABCSG8 (N=1,702), confirming its ability to accurately predict distant recurrence risk over a 10-year period for both node-negative and node-positive breast cancer patients. These trials underscore EndoPredict’s strong ability to identify low-risk patients, allowing for more tailored treatment strategies.
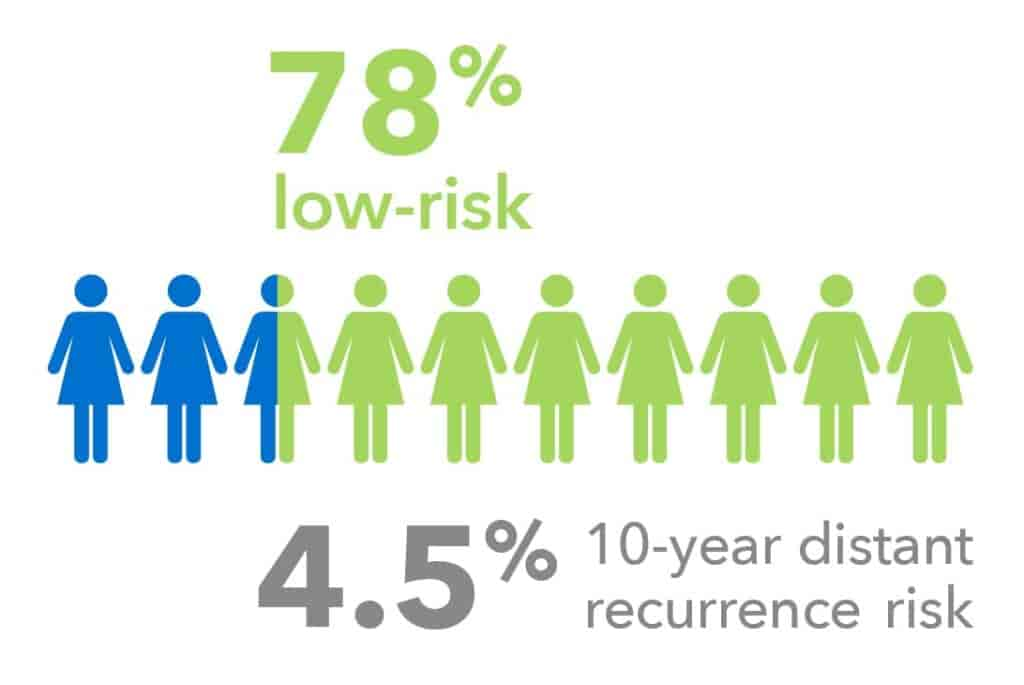
Node-negative Patients
Validated in endocrine-treated women with N0 breast cancer without chemotherapy:
- 78% of node negative patients were identified as low-risk.
- The 10-year distant recurrence risk for patients classified as low-risk by EndoPredict was 4.5%, compared to 13.0% in those classified as high-risk.
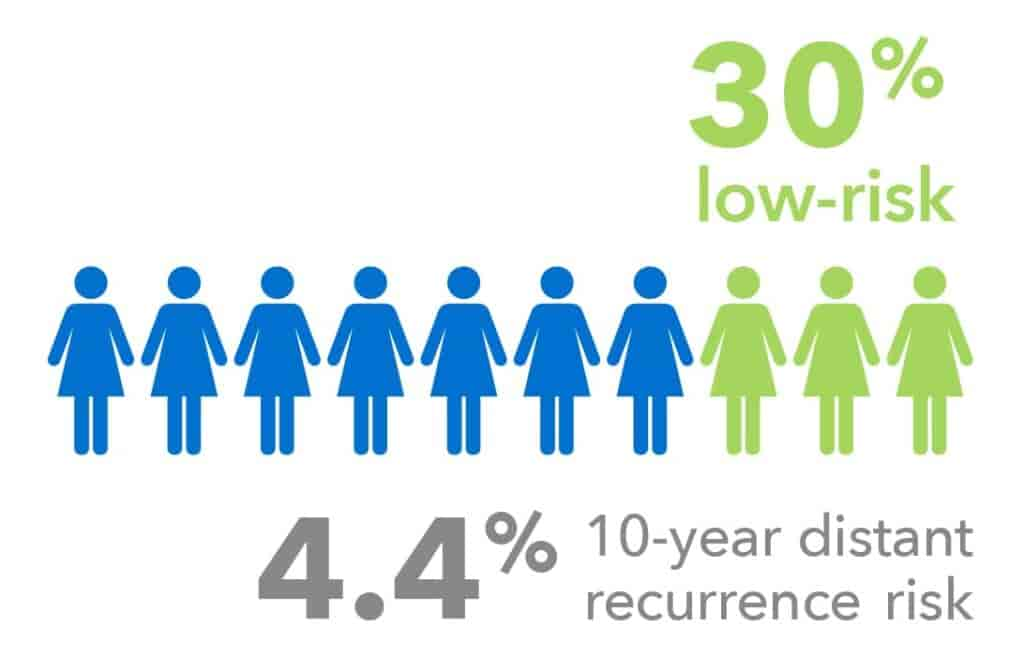
Node-positive Patients
Validated in endocrine-treated women with 1-3 affected lymph nodes without chemotherapy:
- 30% of node positive patients were identified as low-risk.
- The 10-year distant recurrence risk for patients classified as low-risk by EndoPredict was 4.4%, compared to 24.2% in those classified as high-risk.
Prognostic Power Confirmed in Real World Study10
EndoPredict’s prognostic accuracy was further validated in a large prospective real-world cohort (N=842). These findings reinforce its role in refining risk assessments for both node-negative and node-positive breast cancer patients. In clinical practice, the 5-year distant recurrence risk for patients classified as low-risk by EndoPredict was 1.3% in node-negative patients and 2.4% in patients with up to three positive lymph nodes.
By enhancing risk stratification, EndoPredict helps guide treatment decisions, ensuring more personalized and effective care.
Postmenopausal and Premenopausal
Does Menopausal Status Indicate Chemotherapy Need?
While menopausal status plays a role in breast cancer treatment planning, it does not determine individual risk of distant recurrence or chemotherapy benefit. Not all premenopausal patients need chemotherapy. For more accurate and individualized risk assessment, EndoPredict integrates molecular data with key clinical-pathological factors to provide a clearer picture of recurrence risk – regardless of menopausal status.
EndoPredictt® is Validated for Premenopausal and Postmenopausal Breast Cancer Patients7,1
EndoPredict is the only second-generation breast cancer prognostic test validated in both premenopausal and postmenopausal women with ER+, HER2-tumors (pN0–1), confirming its ability to accurately predict distant recurrence risk over a 10-year period for both node-negative and node-positive breast cancer patients.
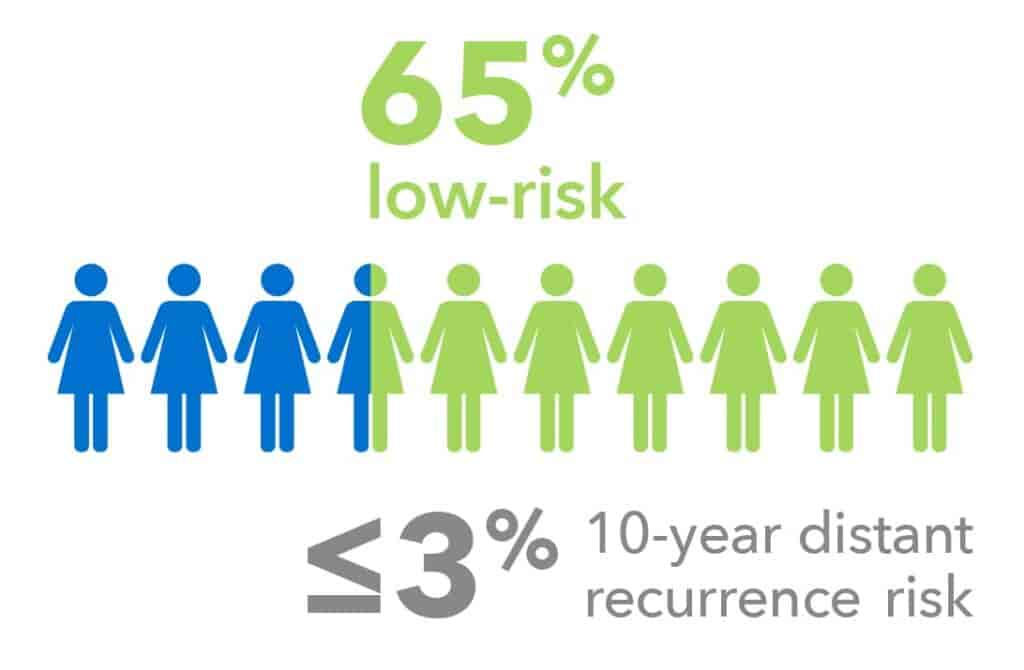
Premenopausal Patients7
Validated in endocrine-treated women without chemotherapy:
- 65% of all premenopausal patients were identified as low-risk.
- 10-year distant recurrence rates of ≤3%, even in patients without ovarian function suppression.
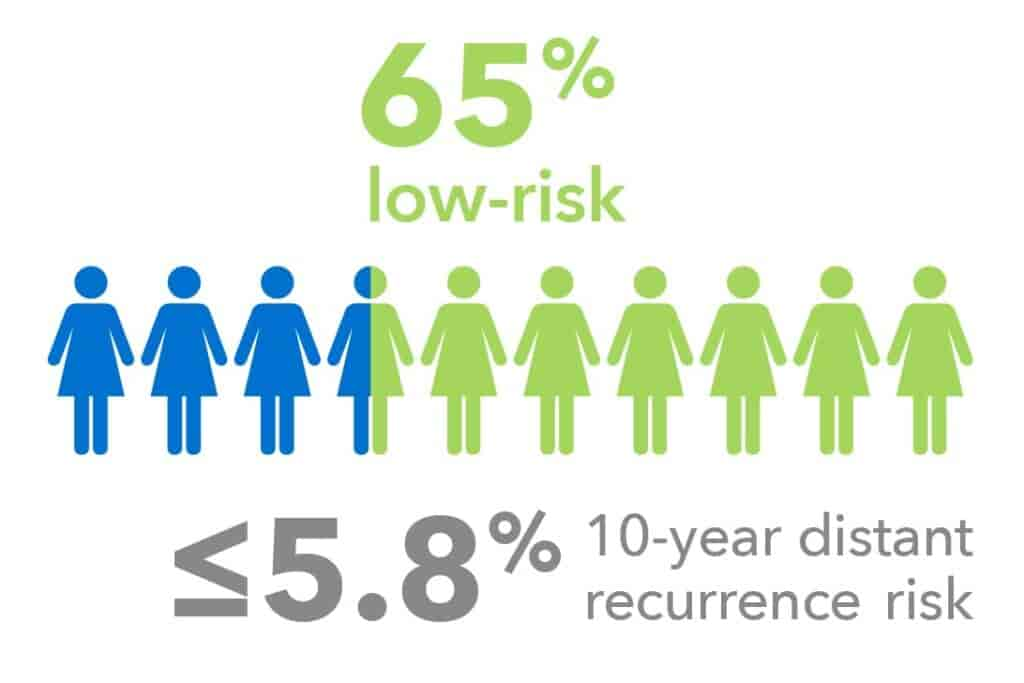
Postmenopausal Patients1
Validated in endocrine-only treated postmenopausal cohorts (e.g., ABCSG8, TransATAC):
- Up to 65% of all postmenopausal patients were identified as low-risk.
- 10-year distant recurrence rates of ≤5.8%.
Prognostic Power Confirmed in Real World Study9
A long-term real-world study with a median follow-up of 8.2 years demonstrated that post- and premenopausal women classified as low-risk by the EndoPredict test experienced consistently low rates of distant recurrence. Premenopausal women classified as low-risk by EndoPredict had a 5-year risk of distant metastasis of just 1.4% , compared to 14.0% in the high-risk group. Among postmenopausal women, the 5-year risk was slightly higher at 4.5% in the low-risk group and 15.9% in the high-risk group.
EndoPredict empowers confident treatment decisions in both pre- and postmenopausal women with ER+/HER2- breast cancer – accurately identifying those who can safely avoid chemotherapy while ensuring high-risk patients receive the care they need.
Evidence You Can Trust
Recommended in major international guidelines, including ASCO, NCCN, AJCC, St. Gallen, and NICE, along with ESMO Level of Evidence 1A recommendation. It also holds a prominent place in national guidelines worldwide.4,11-15
ESMO LoE1A Recommendation for Node Negative and Node Positive Disease4
EndoPredict is recognized in the actual breast cancer guidelines as test for both pre- and postmenopausal patients. The guideline excludes intermediate-risk results – giving EndoPredict a clear advantage with only low- and high-risk categories for confident chemotherapy decisions.
Highest level of evidence (LoE1A)
Pre- and postmenopausal patients
All patients included due to clear results
NICE Recommendations: Trusted in Node-Negative and Node-Positive Breast Cancer15
EndoPredict has been a trusted genomic test since 2018, when NICE first endorsed it for node-negative (N0) and micrometastatic early breast cancer.
In 2024, NICE extended its recommendation to include postmenopausal patients with 1–3 positive lymph nodes, confirming EndoPredict as a reliable tool for guiding chemotherapy decisions.
EndoPredict breast cancer prognostic and chemotherapy predictive test is available for physicians to order via numerous local labs.
- Find your local lab
Choose and contact your local pathology lab from our list of certified laboratories performing EndoPredict. Working with a nearby lab ensures the fastest turnaround time for results.
- Send your sample
Tumor samples from a diagnostic biopsy or surgery specimen (before treatment) can be sent directly to your chosen lab. The laboratory will provide detailed instructions for sample preparation and shipping.
- Results in days
Once the lab has received your sample, results are typically available within a few days—supporting timely, confident treatment decisions for your patients.

References
- Filipits M. et al.: A New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk Factors. Clin. Cancer Res. 2011
- Sestak I. et al.: Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Res Treat. 2019
- Filipits et al.: Prediction of Distant Recurrence using EndoPredict among Women with ER+, HER2- Node- Positive and Node-Negative Breast Cancer Treated with Endocrine Therapy Only. Clin Cancer Res. 2019
- Loibl S. et al.: Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023
- Buus R. et al.: Comparison of EndoPredict and EPclin With Oncotype DX Recurrence Score for Prediction of Risk of Distant Recurrence After Endocrine Therapy. J Natl Cancer Inst. 2016
- Sestak I. et al.: Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer. A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018
- Constantinidou A. et al.: Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2022
- Sestak I. et al.: Prognostic Value of EndoPredict in Women with Hormone Receptor Positive, HER2-Negative Invasive Lobular Breast Cancer. Clin Cancer Res. 2020
- Klein, E. et al.: Long-term outcome data using EndoPredict as risk stratification and chemotherapy decision biomarker in hormone receptor positive, HER2-negative early breast cancer. Breast Cancer Res Treat. 2024
- Schmitt, W. D. et al.: Retrospective evaluation of outcomes in a real-world, prospective cohort using EndoPredict: Results from the Charité registry. SABCS 2022
- Andre F. et al.: Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J Clin Oncol. 2022
- Curigliano G. et al.: Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 2023
- AJCC Cancer Staging Manual- Eight Edition 2017
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer r V.3.2025 ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed [July 17, 2025]. To view the most recent and complete version of the guideline, go online to NCCN.org.
- National Institute for Health and Care Excellence (2024). Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer (Diagnostics guidance [DG58]).