Eurobio Oncology
EndoPredict®: Comparing Tests
Confident Chemotherapy Decisions in Breast Cancer with EndoPredict®
In the management of early ER+, HER2- breast cancer, multigene expression tests can play a pivotal role in guiding adjuvant therapy decisions. But while these tests often appear similar, they differ significantly in what they measure, how results are derived, and how broadly they apply across patient subgroups. These differences can directly affect clinical outcomes.

A Broader Window into Risk and Benefit
Actionable and Personalized Results
EndoPredict combines optimized molecular profiling with essential clinical-pathological data at the core of its risk algorithm.1 This results in:
- More individualized risk and chemotherapy benefit prediction, directly reflecting each patient’s biology and clinical context.1,2
- Clear, fixed cut-off and a binary risk result (low/high)1,3
- Long-term predictive strength, with validated risk estimates at 10 years post-diagnosis, and an estimate of late recurrence risk (years 5–15).1,3
What Each Test Really Tells You
| EndoPredict® | OncotypeDx® | MammaPrint® | Prosigna® | |
| Individual 10-year recurrence risk for all patients | ✓1,4-8 | X9 N0 10 year group risk / N+ 5 year group risk |
X10 5 year group risk |
✓11 No premenopausal indication |
| Individual 10-year Chemotherapy (CTx) benefit validated across studies with over 3.700 patients | ✓2,8 | X9 5 years group benefit / CTx benefit not shown in TailorX and RxPonder for high-risk groups12,13 |
X10 | X11 |
| Individual 5-15 year recurrence risk | ✓3,8 | X9 | X10 | X11 |
| Clear binary risk category | ✓1,3,8 | X9 | ✓ | X11 |
| Consistent cut-off | ✓1,4-7,16-18 | X19,20 | ✓ | X21,22 |
This integrated approach delivers scientifically robust and clinically actionable results – providing confidence in chemotherapy decisions and endocrine therapy planning. By tailoring treatment to each patient’s individual risk profile rather than relying on group averages, it enables truly personalized care.
The clear, meaningful results support shared decision-making and make it easier for patients to understandt heir options and engage in their treatment journey.
Truly Integrated Risk Assessment
EndoPredict is the only assay to fully incorporate tumor size and nodal status within the test itself – automatically factored into the risk algorithm and included in the result report.1,9,10,11

By contrast, other tests do not include clinical-pathological factors directly in their result algorithms – even though it is well known that these factors significantly enhance prognostic accuracy.23-25 Instead of integrating them, the test requires physicians to manually combine molecular results with clinical information using external tools – adding unnecessary complexity and increasing the potential for inconsistent interpretation.
How Test Algorithms Differ
| EndoPredict® | OncotypeDx® | MammaPrint® | Prosigna® | |
| Algorithm include tumor size | ✓1,8 | X9 | X10 | ✓11 |
| Algorithm includes nodal status | ✓1,8 | X9 | X10 | X11 |
With EndoPredict, physicians receive a fully integrated result that includes both molecular and clinical factors8 – saving valuable time and making it easier to communicate clear to patients without relying on external online calculators or added steps.
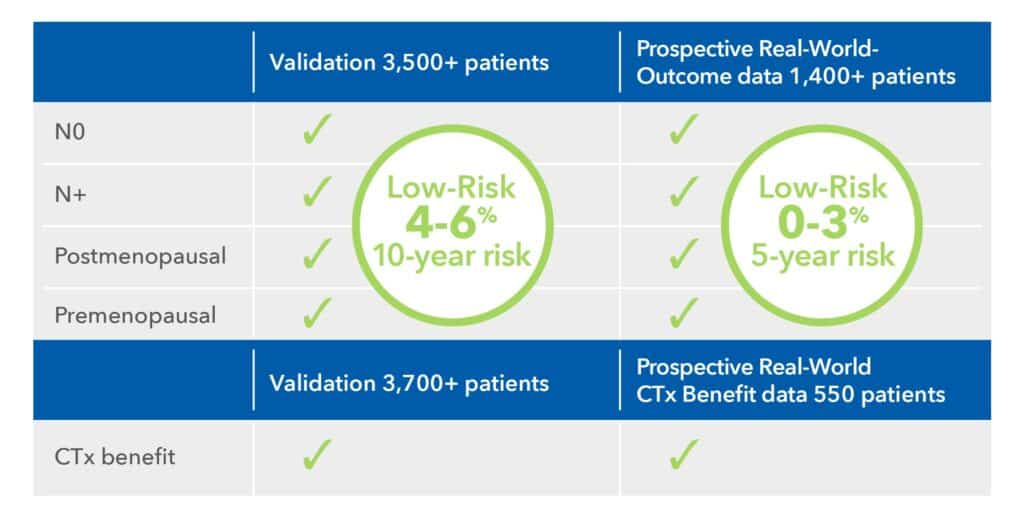
Proven Consistency Across Studies and Subgroups
EndoPredict has demonstrated consistent reliability across a broad range of clinical trials and real-world prospective analyses.1,4-7,16,17 In thousands of patients with ER+, HER2- early breast cancer, it consistently delivers accurate risk stratification across all subgroups:
- Node-negative and node-positive3
- Pre- and postmenopausal women1,3-5-7
- Tumors of various grades and with various Ki67 values26
The test consistently identifies patients with a <10% risk of distant recurrence within 10 years. Its ability to predict chemotherapy benefit is robustly validated1,3,5-7 and confirmed through prospective real-world data.16,17
In contrast, other tests have shown variability across studies. For example, in a real-world cohort of over 28,000 patients tested with OncotyoeDx®:27
- Patients >50 years, N1, RS 20–25 did benefit from chemotherapy
- Patients ≤50 years, N1, RS 0–19 did not
- Both results directly contradict RxPONDER trial findings.12
Such discrepancies raise concerns about the generalizability of Oncotype DX’s cut-offs in certain subgroups – potentially leading to over- or undertreatment.
Value for All Indicated Patients
EndoPredict delivers reliable risk assessment for all ER+, HER2– patients – including node-negative and node-positive, pre- and postmenopausal women. Unlike Oncotype DX®, which lacks clear value in premenopausal node-positive patients12, or Prosigna®, which isn’t indicated for premenopausal women, EndoPredict covers this critical group. It also adds proven value beyond clinical-pathological factors.16,26 In contrast, MammaPrint® offers little additional benefit13, while EndoPredict consistently reclassifies discordant cases with high precision – enabling confident, individualized treatment planning.
Indication and Clinical Value Across Patient Groups
| EndoPredict® | OncotypeDx® | MammaPrint® | Prosigna® | |
| ER+, HER2- early breast cancer N0 and N+ | ✓1,3-5-7 | ✓ | ✓ | ✓ |
| Pre- and postmenopausal | ✓1,3-5-7 | ✓ | ✓ | X22 |
| Pre- and post-surgery | ✓28 | ✓ | ✓ | X22 |
| Value for all indicated patients | ✓1-5.7.16,17,26,29 | X12 Not for N+ premenopausal |
X13,30 No added value to clinical pathological factors |
✓ |
This gives physicians a reliable solution and eliminates uncertainty about test selection for specific subgroups – avoiding uncertainty and ensuring every patient receives the right test from the start.
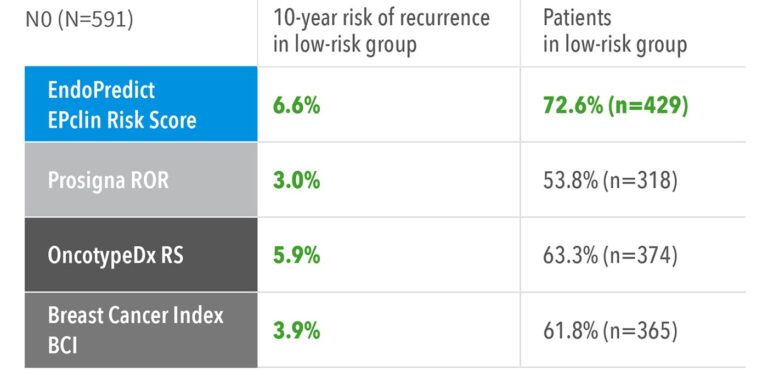
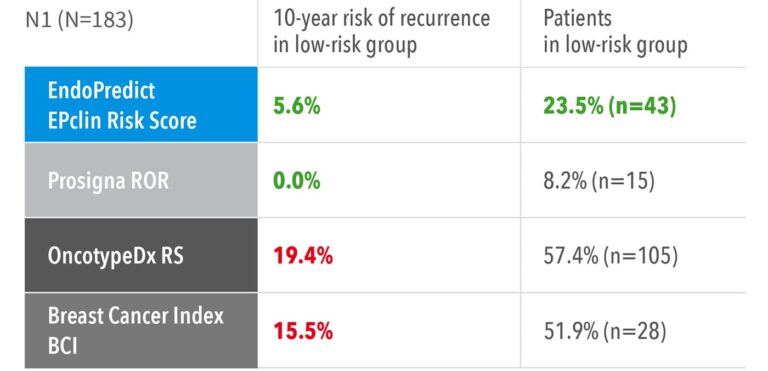
Superior in Identifying True Low-Risk Patients
- Highest sensitivity: EndoPredict demonstrates the highest sensitivity in identifying patients with a distant recurrence risk below 10% over 10 years – classifying the largest proportion as truly low-risk. This enables more patients to avoid unnecessary chemotherapy.5
- Superior performance: In node-positive patients, EndoPredict stands out as one of only two signatures able to define a clinically meaningful low-risk group eligible for endocrine therapy alone – and it identifies the highest number of such patients, offering unmatched confidence in treatment de-escalation.5
Accuracy to identify low-risk patients with node negative and node positive disease from TranATAC5
These results confirm that EndoPredict provides more accurate risk stratification and greater clinical utility, helping physicians confidently spare more patients from unnecessary chemotherapy.
More Insights from Head-to-Head
More Accurate Treatment Decisions for 1 in 4 Patients
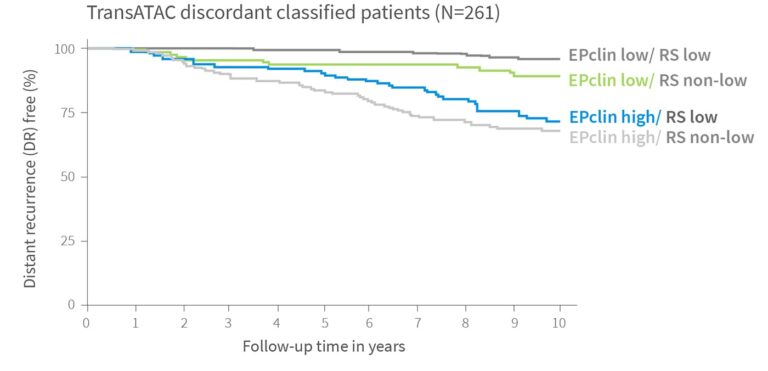
Distant recurrence free survival of ER+ HER2- patients from TransATAC within different risk groups4
EndoPredict outperforms by combining advanced gene expression analysis with key clinical parameters such as tumor size and nodal status – delivering greater prognostic precision than tests based on gene expression data alone. The likelihood ratio shown below is a statistical measure that provides information on how strongly test results are associated with an outcome.
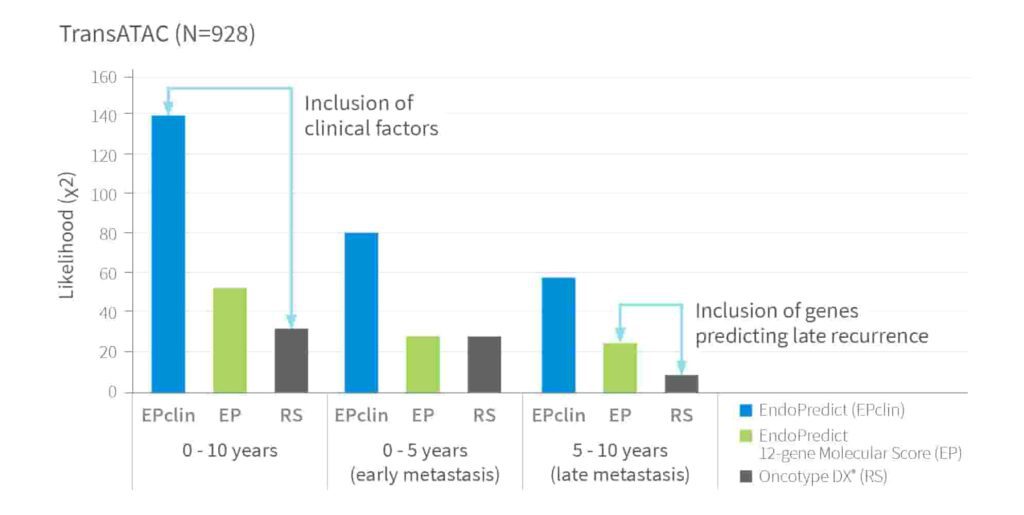
Prognostic ability of EndoPredict, 12-Gene Molecular Score and RS to detect Distant Recurrence4
Sustained Low-Risk Classification - Even in N+ Patients
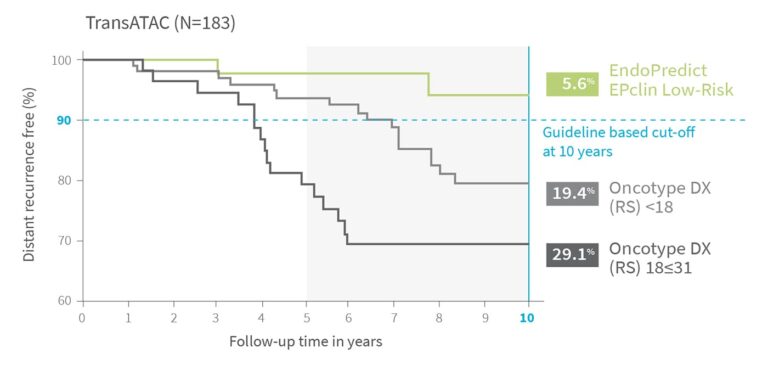
Direct comparison of ‘low-risk’ patients from TransATAC with 1-3 positive lymph nodes4
EndoPredict delivers the most reliable identification of true low-risk patients empowering clinicians and patients with the confidence to make truly personalized, evidence-based treatment decisions.
Fast, Reliable Results – Locally Validated31-33
- Results in just a few days
- Full compliance with regional quality assurance standards
- Smoother integration with standard clinical workflows
- Consistent analytical quality shown in kit validation and round robin trials31-33
Local Testing and Validation
| EndoPredict® | OncotypeDx® | MammaPrint® | Prosigna® | |
| Country testing center network | ✓ | X | X | ✓ |
| Local Validation | ✓31-33 | X | ✓ | ✓ |
In contrast, other tests rely on centralized processing, which can delay results and complicate logistics. While some may operate within limited laboratory networks, this approach is not universally available and may hinder timely treatment decisions.
Decentralized testing – offered by only a few – ensures faster turnaround, reduces patient anxiety through shorter waiting times, and provides clinicians with the confidence of robust local quality control for immediate, informed treatment planning.
Trusted by Experts
ESMO Recognition: Level of Evidence (LoE1A6)
Unlike MammaPrint®, which is not recommended by NICE30, and Oncotype DX®, whose intermediate-risk category was excluded from ESMO recommendations38, EndoPredict delivers consistently high prognostic accuracy with clear results across all patient groups.
Its strong evidence base has made it a trusted tool in the care of thousands of breast cancer patients worldwide – supporting truly personalized treatment decisions.
Highest level of evidence (LoE1A)
Pre- and postmenopausal patients
All patients included due to clear results
References
- Filipits M. et al.: A New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk Factors. Clin. Cancer Res. 2011
- Sestak I. et al.: Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Res Treat. 2019
- Filipits M. et al.: Prediction of Distant Recurrence using EndoPredict among Women with ER+, HER2- Node- Positive and Node-Negative Breast Cancer Treated with Endocrine Therapy Only. Clin Cancer Res. 2019
- Buus R. et al.: Comparison of EndoPredict and EPclin With Oncotype DX Recurrence Score for Prediction of Risk of Distant Recurrence After Endocrine Therapy. J Natl Cancer Inst. 2016
- Sestak I. et al.: Comparison of the Performance of 6 Prognostic Signatures for Estrogen Receptor-Positive Breast Cancer. A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018
- Martin M. et al.: Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− reast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res. 2014
- Constantinidou A. et al.: Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2022
- Result Report EndoPredict
- Result Report Oncotype Dx
- Result Report MammaPrint
- Result Report Prosigna
- Kalinsky K. et al.: 21-Gene Assay To Inform Chemotherapy Benefit In Node-Positive Breast Cancer. N Engl J Med. 2021
- Cardoso F. et al.: 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016
- Knauer M. et al.: The predictive value of the 70-gene signature for adjuvant chemotherapy benefit in early breast cancer. Breast Cancer Res Treat. 2010
- Piccart M. et al.: 70-gene signature as an aid to treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial. Lancet Oncol. 2021
- Klein, E. et al.: Long-term outcome data using EndoPredict as risk stratification and chemotherapy decision biomarker in hormone receptor positive, HER2-negative early breast cancer. Breast Cancer Res Treat. 2024
- Schmitt, W. D. et al.: Retrospective evaluation of outcomes in a real-world, prospective cohort using EndoPredict: Results from the Charitéregistry. SABCS 2022
- Vázquez-Juarez D. et al.: Follow-up of prospective cohort of Mexican premenopausal women with breast cancer who received guided adjuvant treatment with the EndoPredict assay. SABCS 2021
- Paik S. et al.: Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018
- Prosigna® Breast Cancer Prognostic Gene Signature Assay Package Insert (US, 2022)
- Prosigna® Breast Cancer Prognostic Gene Signature Assay Package Insert (English for EU)
- Sparano, J. A. et al.: Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019
- Sparano JA, et al.: Integration of clinical and genomic risk for late distant recurrence in hormone receptor–positive breast cancer. NEJM Evid. 2024
- Lajos T, et al.: RSClin for risk stratification in node-positive, hormone receptor–positive, HER2-negative breast cancer. J Clin Oncol. 2024
- Dubsky P. et al.: EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013
- Stabelini R. et al.: Adjuvant chemotherapy is associated with an overall survival benefit regardless of age in ER+/HER2- breast cancer pts with 1-3 positive nodes and oncotype DX recurrence score 20 to 25: an NCDB analysis. Front Oncol. 2023
- Müller B. M. et al.: Comparison of the RNA-based EndoPredict multigene test between core biopsies and corresponding surgical breast cancer sections. J Clin Pathol. 2012
- Sestak I. et al.: Prognostic Value of EndoPredict in Women with Hormone Receptor Positive, HER2-Negative Invasive Lobular Breast Cancer. Clin Cancer Res. 2020
- National Institute for Health and Care Excellence (2024). Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer (Diagnostics guidance [DG58]).
- Kronenwett R. et al.: Decentral gene expression analysis: analytical validation of the EndoPredict genomic multianalyte breast cancer prognosis test. BMC Cancer. 2012
- Denkert C. et al.: Decentral gene expression analysis for ER+/Her2- breast cancer: results of a proficiency testing program for the EndoPredict assay. Virchows Arch. 2012
- Lehmann-Che J. et al.: First French pilot quality assessment of the EndoPredict test for early luminal breast carcinoma. Anticancer Res. 2018
- Andre F. et al.: Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J Clin Oncol. 2022
- Curigliano G. et al.: Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 2023
- AJCC Cancer Staging Manual- Eight Edition 2017
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.2.2025 ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed [June 6, 2025]. To view the most recent and complete version of the guideline, go online to NCCN.org
- Loibl S. et al.: Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023