Eurobio Oncology
EndoPredict®: Test Procedure
Intended Use1
The EndoPredict® QS Kit is an in vitro diagnostic product for patients with estrogen-receptor-positive, HER2-negative primary breast cancer to determine the risk of distant recurrence and to estimate the adjuvant chemotherapy benefit.
For the intended purpose, the EndoPredict® QS Kit may only be applied to RNA samples extracted from formalin-fixed, paraffin-embedded breast cancer tissue.
The EndoPredict QS Kit is designed for use by experts trained in the assay techniques required for performance of the test and in the application of the system. The results of the EndoPredict test may be used only in the proper context, together with other established methods and clinical/pathological factors for the prognosis and stratification of patients with breast cancer.

- Limitations:
For the intended purpose, the EndoPredict® QS UNO and DUO Kits may only be applied to RNA samples extracted from formalin-fixed, paraffin-embedded breast cancer tissue using any of the following RNA extraction kits or reagents:
- RNeasy® DSP FFPE Kit (QIAGEN®)
- RNeasy® FFPE Kit (QIAGEN® ) with Deparaffinization solution (QIAGEN®)
- miRNeasy FFPE Kit (QIAGEN® ) with Deparaffinization solution (QIAGEN®)
- Maxwell® CSC RNA FFPE Kit (Promega®)
- Maxwell® RSC RNA FFPE Kit (Promega®)
Moreover, the EndoPredict QS UNO and DUO Kits may only be used in combination with the EndoPredict Report Generator and together with the following Real-Time PCR Instrument:
- QuantStudio™ 5 Dx Real-Time PCR System (Thermo Fisher Scientific) and the corresponding EndoPredict template files
Performance characteristics of the EndoPredict test have only been established for women with estrogen-receptor positive, HER2-negative breast cancer treated with either adjuvant endocrine therapy alone or adjuvant endocrine therapy plus chemotherapy after resection. They are not established for patients treated prior to resection with systemic therapy (e.g. chemotherapy or endocrine therapy) or radiation therapy.
How to perform the EndoPredict® test
1. Sampling1
EndoPredict is performed on FFPE tumor tissue from treatment-naïve biopsy or surgical specimens. To ensure high-quality results, the following criteria should be met:
- Tumor content: Minimum of 30% tumor cells in the sample
- Time to fixation: Within 1 hour after excision at room temperature
- Fixation duration: No longer than 3 days in formalin
- Accepted formats: FFPE tumor block or mounted slides
- Storage and shipping: Store and ship specimens at room temperature
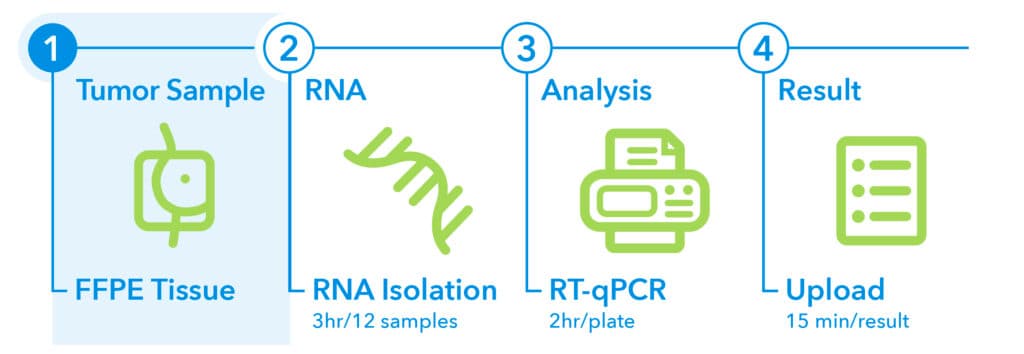
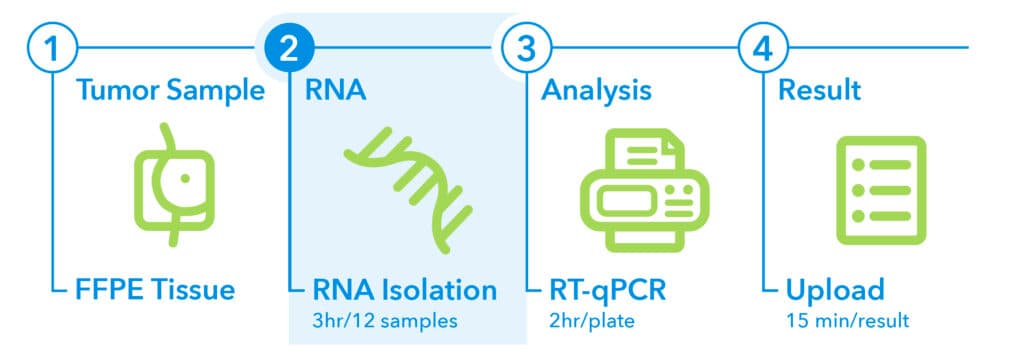
2. RNA Isolation1
RNA extraction can be performed manually or automatically, with a turnaround time of 3 hours per 12 samples. The following RNA extraction kits and reagents are validated for use:
- QIAGEN® RNeasy® DSP FFPE Kit
- QIAGEN® RNeasy® FFPE Kit (used with QIAGEN® Deparaffinization Solution)
- QIAGEN® miRNeasy® FFPE Kit (used with QIAGEN® Deparaffinization Solution)
- Promega® Maxwell® CSC RNA FFPE Kit
- Promega® Maxwell® RSC RNA FFPE Kit
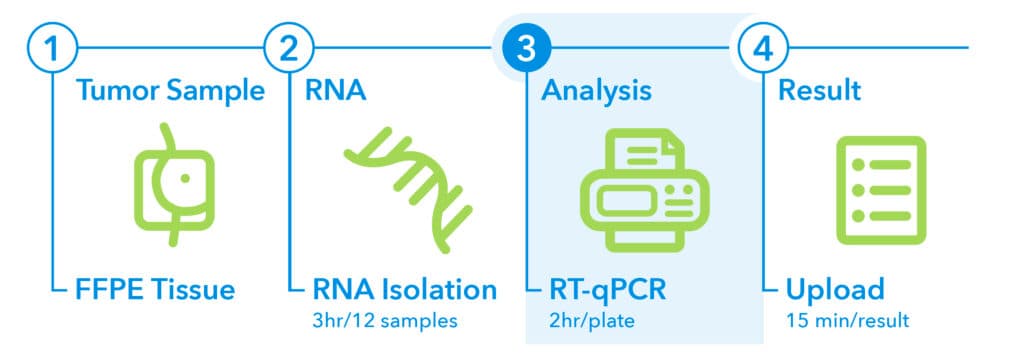
3. Analysis1
- The EndoPredict UNO plate is designed for the analysis of one tumor sample.
- The EndoPredict DUO plate allows for the parallel analysis of two tumor samples.
- The RT-qPCR run time is approximately 1.5 hours.
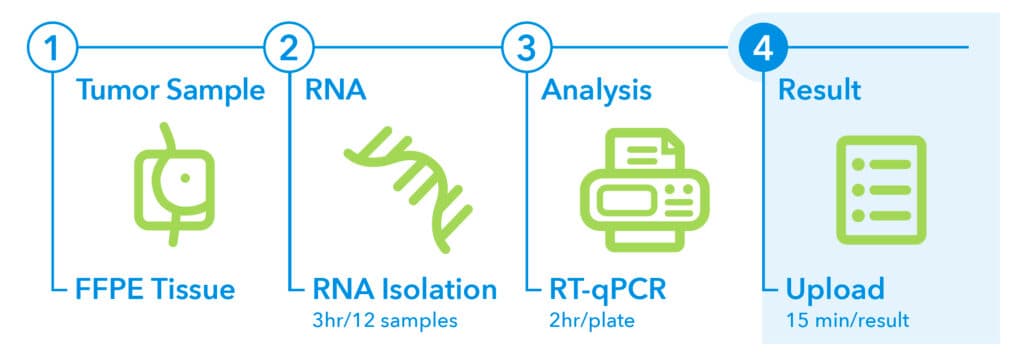
4. Result1
- Two reports (final esult report and summary report) can be created.
- The reports are generated in less than 15 minutes.
How to Order an EndoPredict® Test
The EndoPredict prognostic and predictive breast cancer test is performed in certified local laboratories, delivering reliable results within just a few days.
Physicians can conveniently order EndoPredict through a wide network of participating labs. Use the link below to find a local lab and place your order. Have questions? Contact us anytime – we’re here to help.

Get the EndoPredict® Test in Your Lab
EndoPredict is available as a CE-marked IVD kit that can be run in local molecular pathology laboratories after a comprehensive onsite training, provided by Eurobio Scientific.
Interested in offering EndoPredict in your lab? Use the contact form below – we’ll evaluate the requirements together.
References
- EndoPredict® QS for QuantStudio™ 5 Dx Instruction Manual Version EN/03 2025
