Eurobio Oncology
EndoPredict®: Distant Recurrence Risk Prediction
Guide Early Breast Cancer Treatment Decisions with EndoPredict® - Predicting Risk of Distant Recurrence up to 15 Years
EndoPredict is a second-generation breast cancer prognostic test that accurately predicts the 10-year risk1 and 5-15 years risk2 of distant recurrence, helping clinicians and patients make informed therapy decisions with confidence.
What is the Risk of Breast Cancer Distant Recurrence within
10 Years?
Precise prediction of 10-year distant recurrence risk is essential for guiding early ER+/HER2- breast cancer treatment decisions and ensuring patients receive the most appropriate care. EndoPredict provides this critical insight for pre- and postmenopausal patients, with or without affected lymph nodes1-3, helping clinicians personalize treatment strategies and avoid both over- and under-treatment.
- Independently Validated for Accurate 10-Year Risk Assessment
EndoPredict was validated in four similar prospective retrospective studies providing LoE1.4 The EndoPredict development process involved homogeneous populations from training to validation cohorts: ER+, HER2-, node-negative (N0) and node-positive (N+), pre- and postmenopausal.2,3,5
A dedicated development process focusing only on ER+/HER2- cohorts led to strong performance of the test using always the same score and cut-off values since its inception.
Identifying a Large and True Low-Risk Patient Group
The patients in the ABCSG-6, ABCSG-8, TransATAC and premenopausal studies were treated with endocrine therapy alone. All patients received only 5 years of hormone therapy. In all validation studies, EndoPredict identified a large low-risk group for recurrent breast cancer after 10 years, with a recurrence risk of less than 10%, regardless whether the patients were pre- or postmenopausal.1,3,5
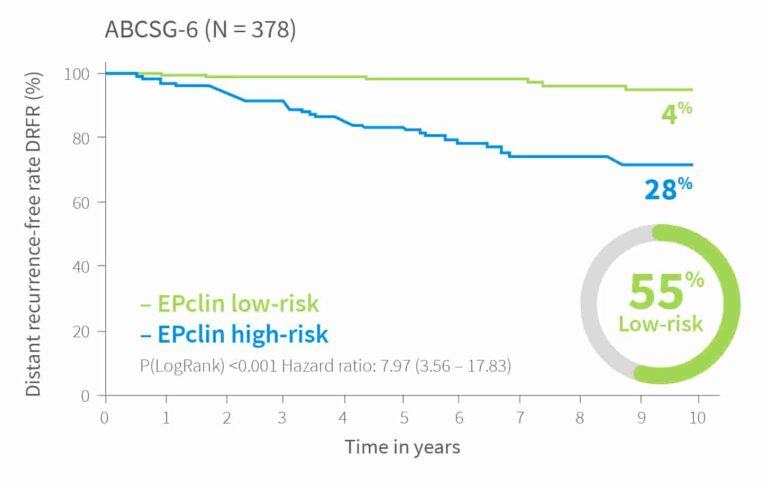
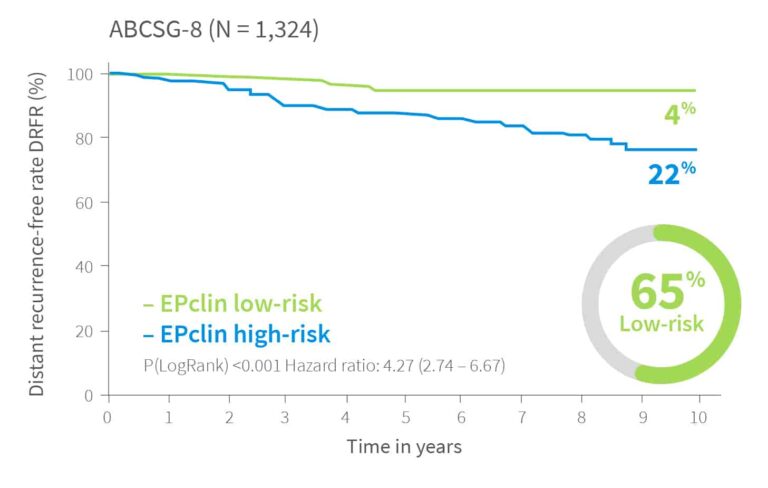
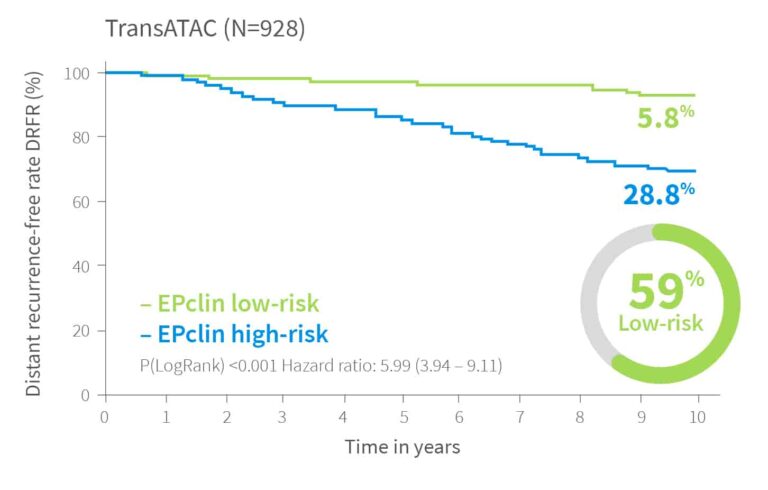
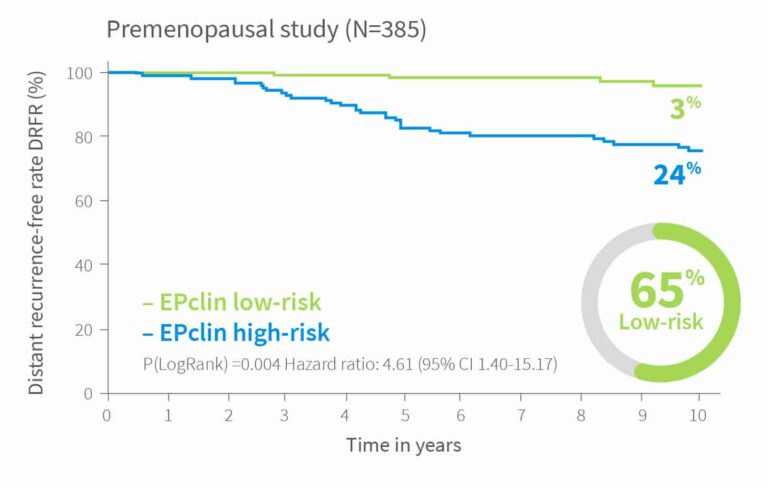
10-year risk by EPclin Risk Score in ER+, HER2-, N+ and N0 patients from ABCSG-6, ABCSG-8 and TransATAC (postmenopausal) and Bank of Cyprus Oncology Centre, Cyprus and the Nottingham University Hospitals NHS Trust, UK (premenopausal)1,3,5
More details on EndoPredict training and validation studies
| Trial | # of patients | % of low-risk group | Breast cancer sub-type | Nodal status | Treatment | Menopausal status | 10-years distant metastasis rate low-risk group | |
| Training | Multicenter1 | 964 | 50% | ER+/HER2- | NO, N+ | 5 years endocrine treatment | Pre + Post | 7% |
| Validation I | ABCSG-61 | 378 | 55% | Post | 4% | |||
| Validation II | ABCSG-81 | 1,324 | 65% | Post | 4% | |||
| Validation III | TransATAC5 | 928 | 59% | Post | 5.8 % | |||
| Validation IV | Premenopausal3 | 385 | 65% | Pre | 3% |
EndoPredict training and validation studies I 1,3,5
- Phase III Evidence Confirms Prognostic Power
EndoPredict is the first second-generation gene expression test to achieve prospective Phase III validation, confirming its strong prognostic performance. With a median follow-up of 70 months, this validation underscores the test’s ability to reliably guide treatment decisions.6 A comparison between the GEICAM/9906 validation study7 and the prospective, randomized Phase III UNIRAD trial6 highlights the reliability of EndoPredict. Both studies included pre- and postmenopausal patients with node-positive, clinically high-risk disease. Patients received endocrine therapy plus chemotherapy, and in both studies, EndoPredict demonstrated a clear and significant separation between high- and low-risk groups.
| Trial | # of patients | % of low-risk group | Breast cancer sub-type | Nodal status | Treatment | Menopausal status | 10-years distant metastasis rate low-risk group | |
| Validation V | GEICAM / 99067 | 555 | 13% | ER+/HER2- | N+ | E + CTx | Pre + Post | 0 % (10 years) |
| Validation VI | UNIRAD6 | 767 | 14% | 0 % (5 years) |
EndoPredict validation studies II 6,7
These consistent results reinforce EndoPredict’s reliability – even in higher-risk populations – offering confidence in treatment decisions for both physicians and patients.
- Prospective Real-World Data Confirm EndoPredict® Accuracy
| Trial | # of patients | % of low-risk group | Breast cancer sub-type | Nodal status | Treatment | Menopausal status | Distant metastasis rate low-risk group |
| TUM8 | 368 | 65% | ER+/HER2- | NO, N1 | Low-risk E 99% | Pre + Post | 3.4% (5 years) |
| Charité9 | 842 | 50% | Low-risk E | Pre + Post | 1.6% (5 years) | ||
| Maxico10 | 99 | 47% | Low-risk E 85% | Pre | 0% (4 years) | ||
| Korean11 | 207 | 74% | Low-risk E 99% | Pre + Post | 0% (5 years) |
Outcome results from real-world registries8-11
The test identifies a high number of low-risk patients with an exceptionally low recurrence risk within five years, helping to guide more personalized treatment decisions in clinical practice.
- Prognostic Value Beyond Traditional Factors
EndoPredict delivers independent prognostic information that goes beyond standard clinicopathologic markers like nodal status, tumor grade, or Ki-67. This added insight supports more precise risk assessment and treatment planning.
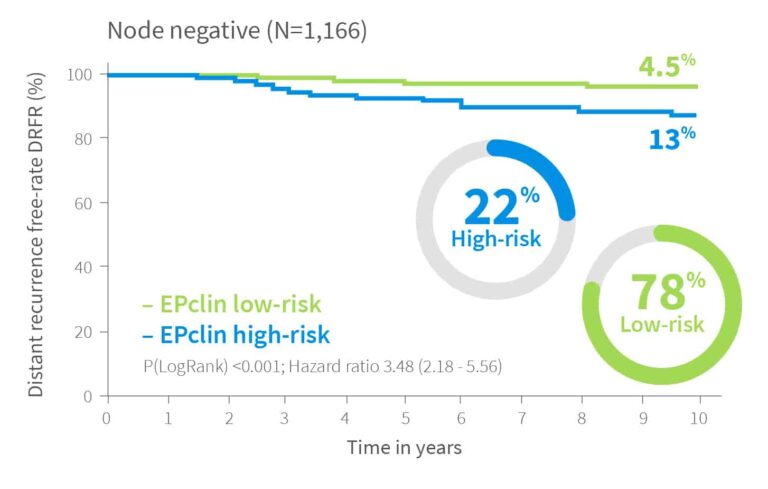
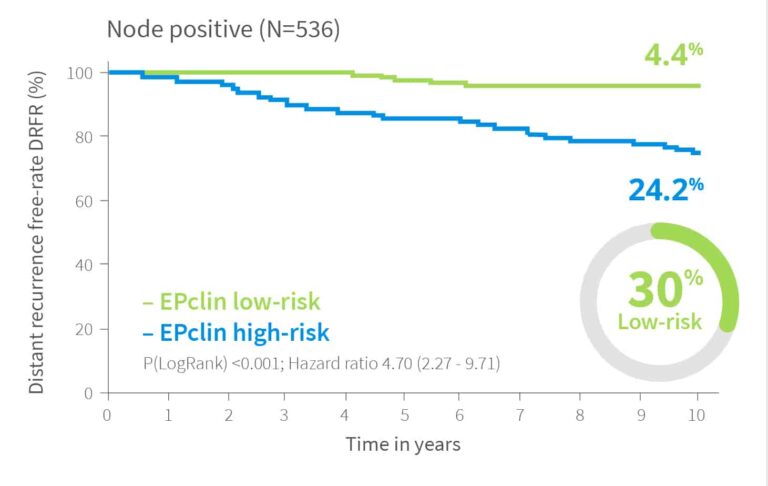
Risk Assessment Independent of Nodal Status
EndoPredict provides meaningful risk stratification regardless of nodal status.2
In validation studies, up to 78% of node negative patients were classified as low-risk, while 22% were identified as high-risk, potentially benefiting from more aggressive treatment. Among node positive patients, up to 30% were reclassified as low-risk, supporting decisions to avoid chemotherapy.
10-year risk by EPclin Risk Score in ER+, HER2-, N0 or N+ from both ABCSG-6 and ABCSG-82
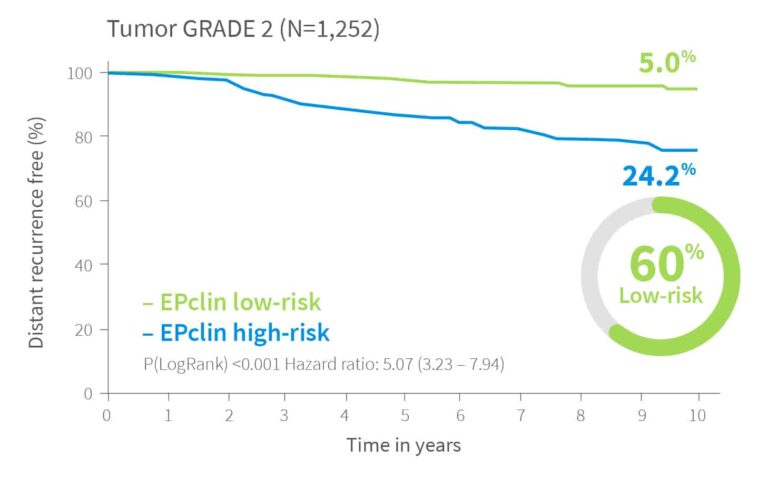
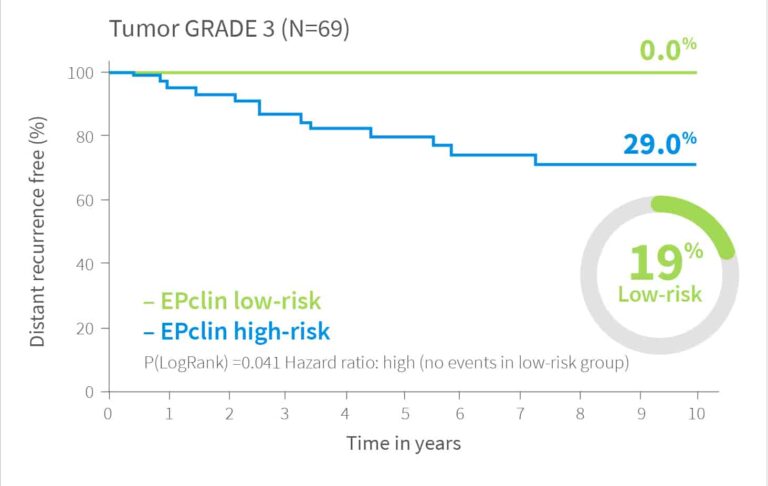
Risk Assessment Independent of Tumor Grade
EndoPredict delivers reliable risk stratification regardless of tumor grade.12
In validation studies, it identified 22% of grade 1 tumors as high-risk and 19% of grade 3 tumors as low-risk.
For grade 2 tumors, EndoPredict classified 60% as low-risk, with a distant recurrence risk below 10% – offering crucial guidance in this often uncertain group.
10-year risk by EPclin Risk Score in ER+, HER2- patients from both ABCSG-6 and ABCSG-8112
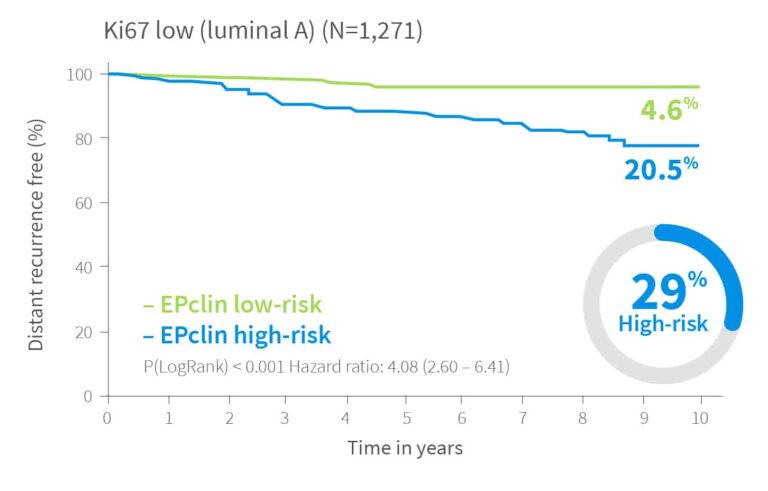
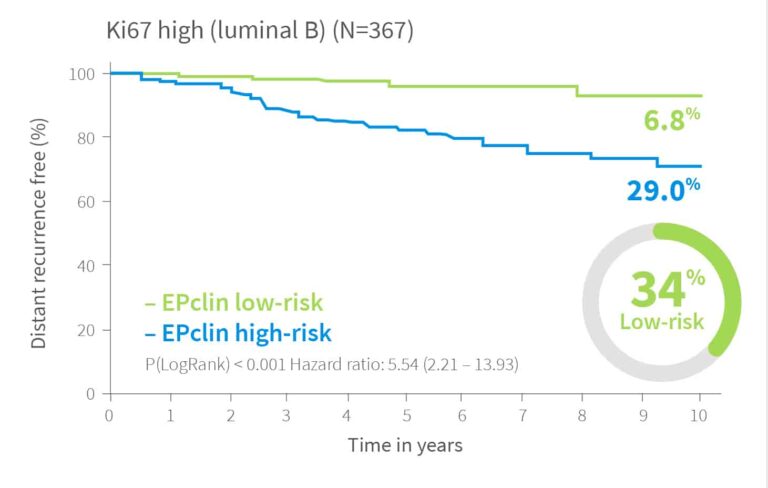
Risk Assessment Independent of Ki-67–Based Luminal Classification
Beyond Prognostic Value
EndoPredict not only improves risk stratification beyond clinical-pathological factors, but also predicts chemotherapy benefit within 10 years.
EndoPredict® Predicts up to 15 Years - Why Long-Term Risk Assessment Matters
In estrogen receptor-positive breast cancer, the risk of recurrence doesn’t end after 5 years. In fact, recurrences continue steadily – even beyond 20 years after diagnosis. More than half of all recurrences in ER+ patients occur after the initial 5-year period.13
That’s why assessing the risk of late recurrence is essential. It helps identify which patients may benefit from extended endocrine therapy beyond the standard 5 years, ensuring more personalized and effective long-term treatment strategies.
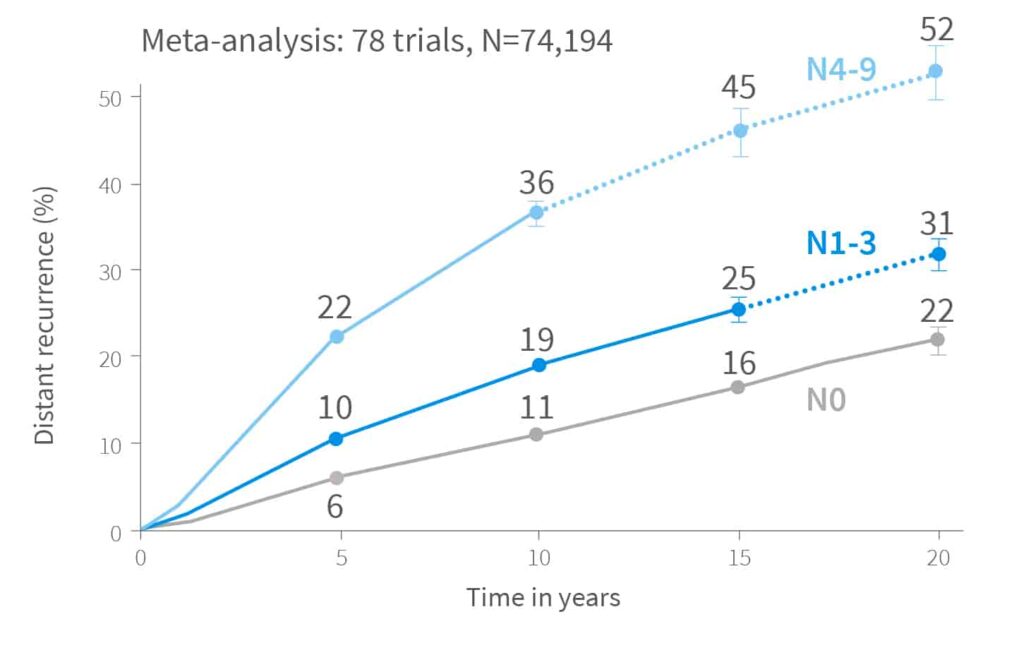
What Is the Risk of Breast Cancer Distant Recurrence Beyond 10 Years?
EndoPredict provides a validated 5 -15-year distant recurrence risk directly in the result report for both node-negative and node-positive ER+/HER2- patients.
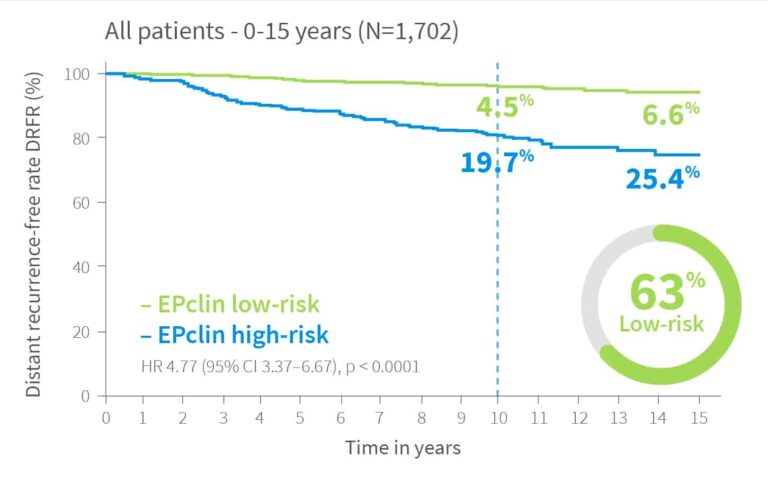
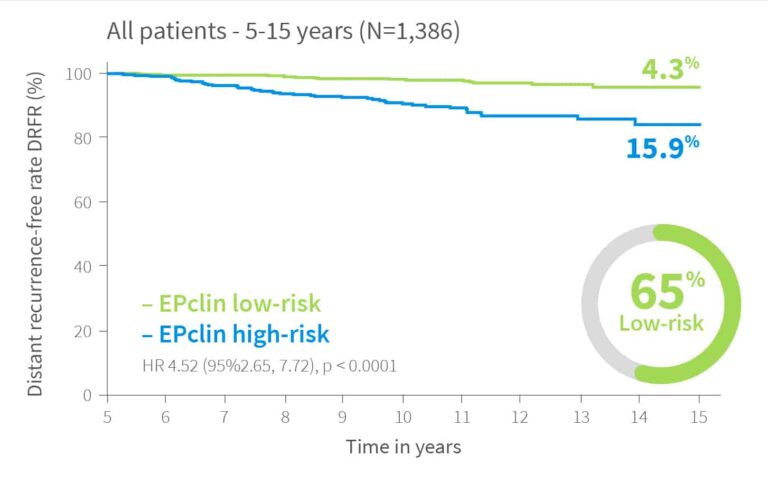
In a re-analysis of the ABCSG6 and ABCSG8 studies2 with up to 16.6 years of follow-up, EndoPredict remained strongly prognostic for late recurrence (years 5–15) in patients who were recurrence-free at year 5.
Patients with a high score had a 4.5-fold higher risk of late distant recurrence compared to those with a low score.
These data support more confident decisions on whether to extend endocrine therapy beyond 5 years.
Risk of distant recurrence by EPclin Risk Score in years 0-10, 0-15 and for patients with no recurrence in first 5 years 5-15 years2
EndoPredict® Testing – Fast, Local, Reliable
The EndoPredict prognostic and predictive breast cancer test is performed in certified local laboratories, delivering reliable results within just a few days.
Physicians can conveniently order EndoPredict through a wide network of participating labs.
Use the link below to find a local lab and place your order. Have questions? Contact us anytime – we’re here to help.

References
- Filipits M. et al.: A New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk Factors. Clin. Cancer Res. 2011
- Filipits M. et al.: Prediction of Distant Recurrence using EndoPredict among Women with ER+, HER2- Node- Positive and Node-Negative Breast Cancer Treated with Endocrine Therapy Only. Clin Cancer Res. 2019;25:3865-3872
- Constantinidou A. et al.: Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2022
- Simon R. M. et al.: Use Of Archived Specimens In Evaluation Of Prognostic And Predictive Biomarkers. J Natl Cancer Inst. 2009
- Buus R. et al.: Comparison of EndoPredict and EPclin With Oncotype DX Recurrence Score for Prediction of Risk of Distant Recurrence After Endocrine Therapy. J Natl Cancer Inst. 2016
- Penault-Llorca F. et al.: Prognostic value of EndoPredict test in patients with hormone receptor positive, human epidermal growth factor receptor 2-negative primary breast cancer screened for the randomized, double-blind, phase III UNIRAD trial. ESMO Open 2024
- Martin M. et al.: Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− reast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res. 2014
- Klein, E. et al.: Long-term outcome data using EndoPredict as risk stratification and chemotherapy decision biomarker in hormone receptor positive, HER2-negative early breast cancer. Breast Cancer Res Treat. 2024
- Schmitt, W. D. et al.: Retrospective evaluation of outcomes in a real-world, prospective cohort using EndoPredict: Results from the Charité registry. SABCS 2022
- Vázquez-Juarez D. et al.: Follow-up of prospective cohort of Mexican premenopausal women with breast cancer who received guided adjuvant treatment with the EndoPredict assay. SABCS 2021
- Jung W. et al.: Treatment Outcomes according to the EndoPredict Score in ER-Positive, HER2-Negative Early Breast Cancer. Breast Care 2022
- Dubsky P. et al.: EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013; 24:640-647
- Pan H. et al.: 20-Year Risks Of Breast-Cancer Recurrence After Stopping Endocrine Therapy At 5 Years. N Engl J Med. 2017; 377(19):1836-1846