Eurobio Scientific becomes one of the first European companies to obtain
IVDR1 CE marking of PCR tests
Eurobio Scientific (FR0013240934, ALERS, PEA-PME eligible), a leading French group in in vitro
specialty medical diagnostics and life sciences, announces today that it obtained in April 2023 the
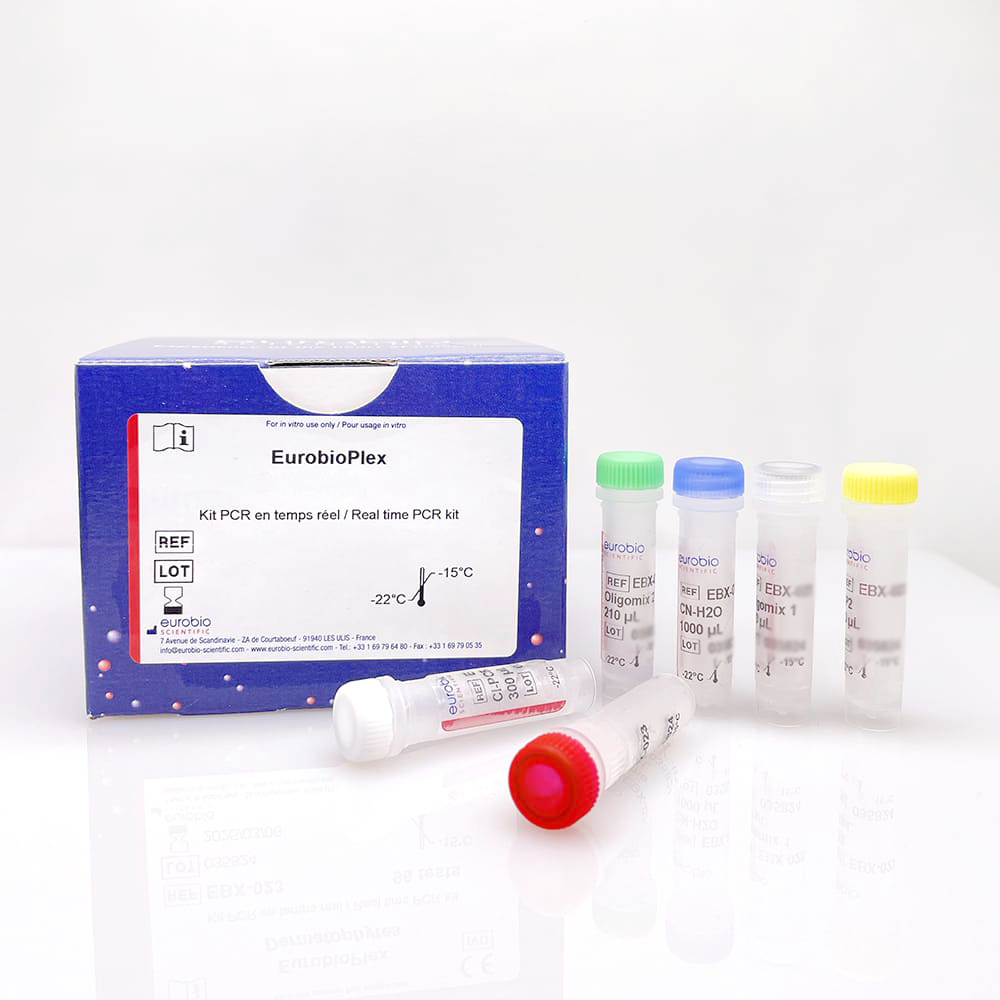
first IVDR CE markings for PCR tests from its EurobioPlex line, thus becoming one of the first
European companies to obtain such marking for its own products.
IVDR CE marking of PCR tests
As part of the new IVDR regulation, Eurobio Scientific has received IVDR CE markings that allow the
marketing of the first 3 PCR tests from its EurobioPlex line as class C in-vitro diagnostic medical
devices, requiring expertise in molecular diagnostics of infectious diseases.
The other tests in the EurobioPlex line, which were already CE marked according to the old regulation
(Directive 98/79/EC) before May 26, 2022, benefit from an extension period which allows them to
be marketed until obtaining their IVDR CE marking which must take place no later than May 2025
for class D devices (diseases presenting a high risk, HIV, hepatitis, etc.), May 2026 for class C, and
May 2027 for class B and A sterile.
Achievement of IVDR ISO certification
This CE marking follows the company’s annual audit by its French notified body, GMED, during which
its ISO 13485:2016 certification was renewed for 3 years (certificate N°39268 rev. 0) with a quality
management system that complies with the requirements of regulation (EU) 2017/746 – IVDR which
imposes new, more restrictive rules for maintaining the certification and CE marking of products. This
system covers all operational aspects of Eurobio Scientific, in France, related to the processes and
procedures that ensure the quality and performance of the PCR tests produced by the company, a
mandatory prerequisite to comply with the new IVDR requirements.
This ISO certification was obtained for “in-vitro diagnostic devices: kits, reagents and control materials
intended to be used to detect the presence of an infectious agent or exposure to such an agent,
including sexually transmitted agents”. It therefore allows Eurobio Scientific to proceed sequentially
with the new CE marking of its current infectious disease PCR tests, and to CE mark its future tests
within the IVDR framework which now applies to all new in-vitro diagnostic medical devices.