Eurobio Scientific
EurobioPlex
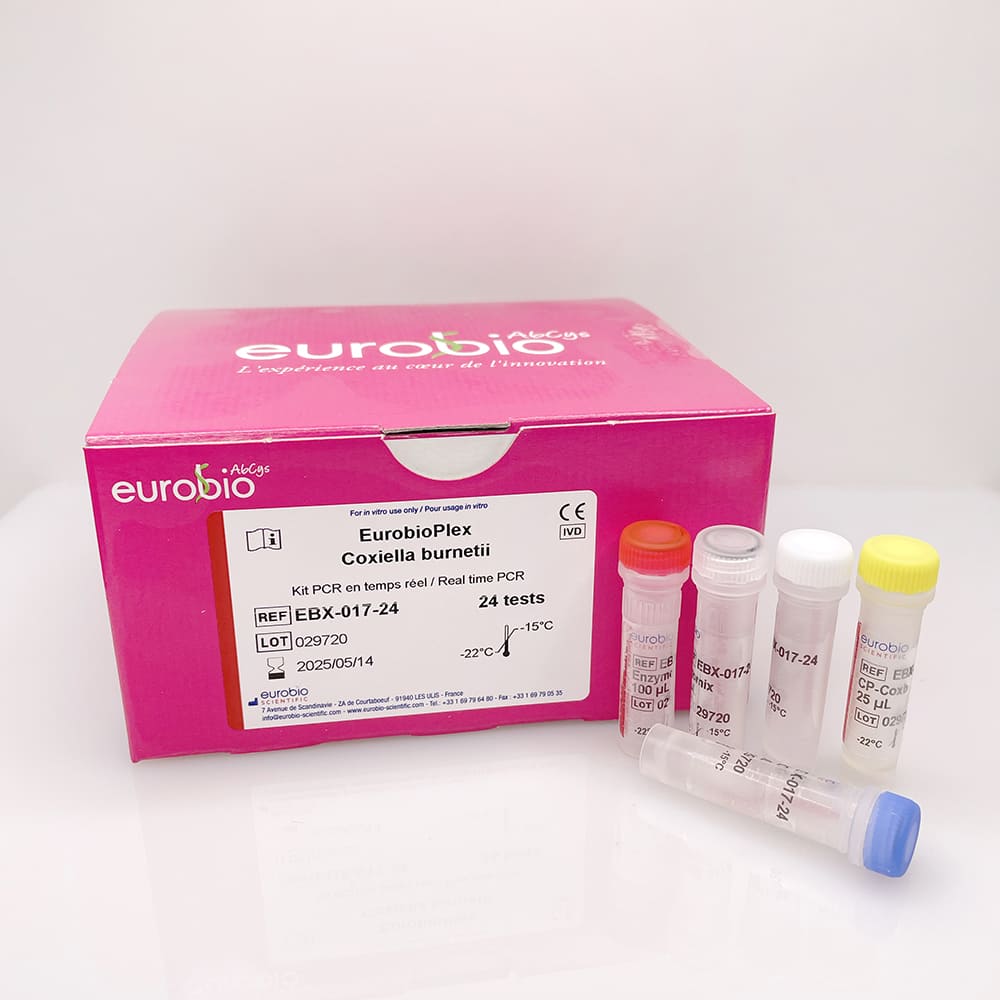
EurobioPlex Coxiella burnetii
The EurobioPlex Coxiella burnetii test uses real time polymerase chain reaction (PCR) amplification and is designed for the qualitative detection of Coxiella burnetii from a DNA nucleic acids extract. This test is indicated to confirm a diagnosis of presumption of infection in patients or complete a proven or indeterminate diagnosis.
- Coxiella burnetii / CI-PCR
- CE-IVD
- Packaging:24, 48, 96 tests
- Qualitative
- Serum, Culture, Blood, Cardiac biopsy, Pharyngeal biopsy, Bone biopsy, Puncture fluid, Lymph node biospy, Biopsy of aneurysmal cell
You have questions or want more informations?
- REF : EBX-017
- Pathogens : Coxiella burnetii / CI-PCR
- Shipment : Dry ice
- Storage at -20 °C
- EurobioPlex Thermocycleur : CFX96™Real Time PCR detection system (Bio-Rad), LightCycler® 480 Instrument II (Roche), Applied Biosystems® 7500 Real Time PCR Systems (Applied Biosystems), T-COR8™-IVD (Tetracore Inc)
- EurobioPlex Extractor : DNA extraction Suitable for Respiratory specimens
| Coxiella burnetii | |
| Analytical Sensitivity on CFX96 in copies /µl | 10 |
| Clinical sensitivity | > 99% |
| Clinical specificity | > 99% |
You may also like
Related products
-
Bacteriology
EurobioPlex Strepto B
-
EurobioPlex
EurobioPlex Monkeypox Screening Test II
Our Products & Services
For the field of transplantation, the group produces reagents and software for transplant matching and chimerism monitoring and culture media for cornea graft transplants.
Eurobio Scientific manufactures prognostic genetics tests for breast cancer and prostatic cancer.
The group develops and markets genetic molecular tests to detect a wide variety of illnesses, including detecting infectious diseases, fertility testing or assaying dermatological conditions.
To support the research and development field, Eurobio Scientific has a broad portfolio in Life Sciences products including cell culture media and quality controls.